Tuesday , February 4, 2020 from 6 p.m to 8 p.m.
College Readiness Math:
| Dimensional Analysis |
Dimensional Analysis (also called Factor-Label Method or the Unit Factor Method) is a problem-solving method that uses the fact that any number or expression can be multiplied by one without changing its value. It is a useful technique. The only danger is that you may end up thinking that chemistry is simply a math problem - which it definitely is not.
Unit factors may be made from any two terms that describe the same or equivalent "amounts" of what we are interested in. For example, we know that
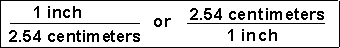
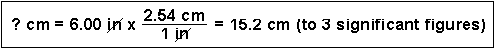
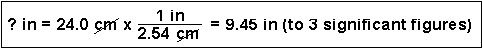
(3) How many seconds are in 2.0 years?
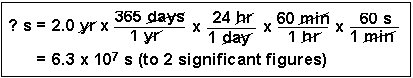
| Prefix | Abbreviation | Meaning | Example |
|---|---|---|---|
| mega- | M | 106 | 1 megameter (Mm) = 1 x 106 m |
| kilo- | k | 103 | 1 kilogram (kg) = 1 x 103 g |
| centi- | c | 10-2 | 1 centimeter (cm) = 1 x 10-2 m |
| milli- | m | 10-3 | 1 milligram (mg) = 1 x 10-3 g |
| micro- | 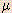 | 10-6 | 1 micrometer (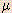 g) = 1 x 10-6 g) = 1 x 10-6 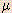 g g |
| nano- | n | 10-9 | 1 nanogram (ng) = 1 x 10-9 g |
(4) Convert 50.0 mL to liters. (This is a very common conversion.)
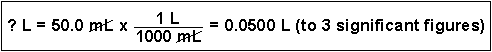
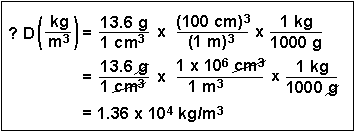
(6) How many atoms of hydrogen can be found in 45 g of ammonia, NH3?
We will need three unit factors to do this calculation, derived from the following information:
- 1 mole of NH3 has a mass of 17 grams.
- 1 mole of NH3 contains 6.02 x 1023 molecules of NH3.
- 1 molecule of NH3 has 3 atoms of hydrogen in it.

QUIZ:
| Question 1 | How many millimeters are present in 20.0 inches? |
| Question 2 | The volume of a wooden block is 6.30 in3. This is equivalent to how many cubic centimeters? |
| Question 3 | A sample of calcium nitrate, Ca(NO3)2, with a formula weight of 164 g/mol, has 5.00 x 1027 atoms of oxygen. How many kilograms of Ca(NO3)2 are present? |
Answers: (1) 508 mm (2) 103 cm3 (3) 227 kg
No comments:
Post a Comment